Comecer and the principle of Safety First
Responsibility as core business in the Life Sciences sector.
In recent years, many companies have embraced the concept of sustainability and responsibility as key elements of business development and, often, as marketing levers. Foundations, funding for social causes, inclusion programs, and a growing focus on environmental impact have become common in the business landscape. However, few companies make the protection of health and safety of operators the focus of an innovation drive and the center of their economic interests. In the pharmaceutical and medical technology landscape, Comecer is certainly among these.
Fifty years of history, present in over 80 countries

With a history starting in 1975, Comecer has gone through decades of technological evolution, transforming from a supplier for the Italian Nuclear Agency into a global leader in the field of aseptic processing and containment in the pharmaceutical and nuclear medicine industries.
Specializing in isolation solutions for regenerative medicine and tissue engineering, Comecer also designs and manufactures high-tech systems through the development of sustainable, safe solutions that always comply with GMP (Good Manufacturing Practice) regulations.
While Comecer's heart beats in Italy, its vision is international with a presence in over 80 countries. Since 2019, it has become part of the ATS Corporate family, and more recently, it has been integrated into the ATS Life Sciences Group division, which brings together all the ATS group companies operating in the health and wellness sector, creating synergy among products and technologies. Additionally, continuing its policy of expansion, Comecer has grown through targeted acquisitions, such as that of DF Siena in 2021.
The governance structure of Comecer SpA, based on transparency and efficiency, consists of three bodies (the Shareholders' Meeting, the Board of Directors, and the Board of Statutory Auditors), while the Organizational Model 231, adopted in 2012, and the "Safety First" Ethical Code are the pillars of the corporate culture, reflecting a commitment to all those ethical principles that guide the company's operations, from constant vigilance over processes and activities to the responsible choice of suppliers and external collaborators.
Innovation, versatility, and an unconditional commitment to safety
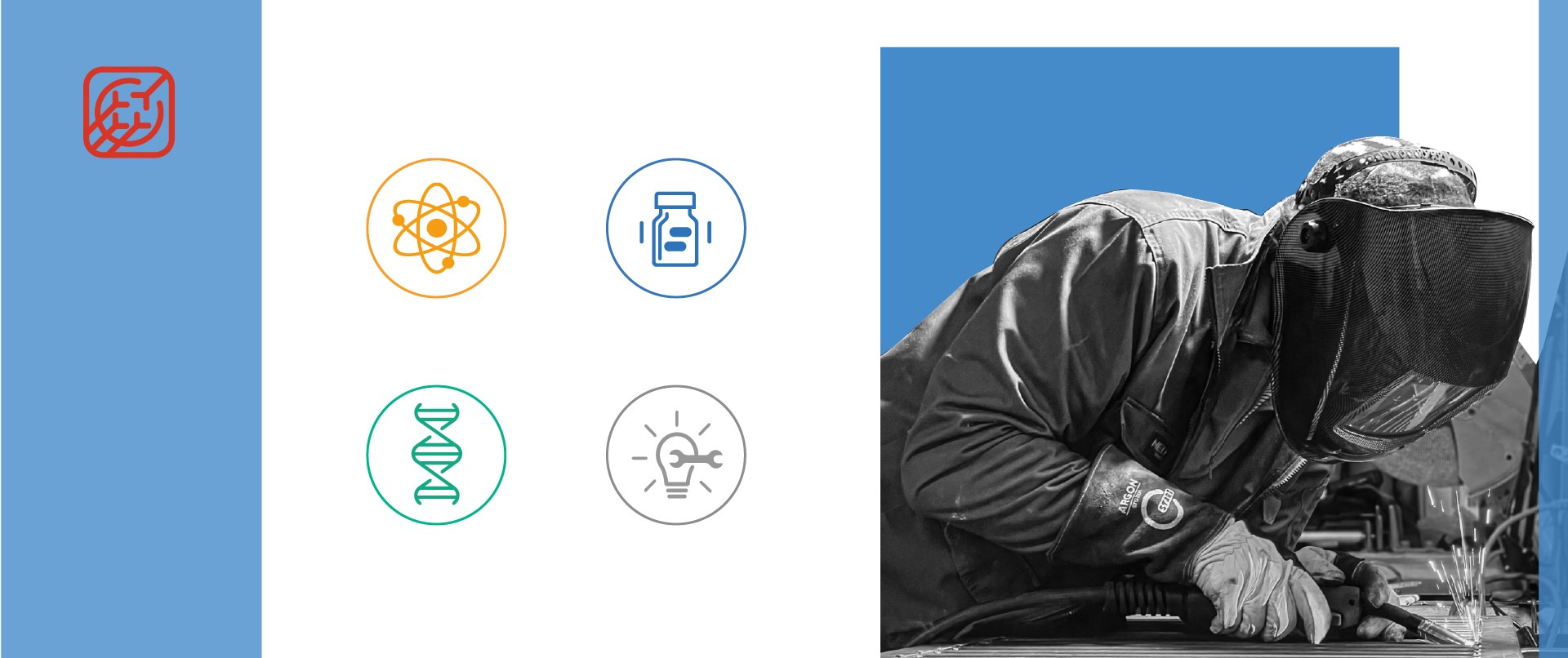
Today, Comecer is a versatile company operating in different sectors and adapting to different markets.
1. In the Radiopharmaceutical sector, Comecer is a leader in the production of systems for the safe handling of radioactive substances used in Nuclear Medicine.
2. In the Pharmaceutical sector, the company specializes in Isolation Technology. In this area, Comecer produces customized isolators that provide maximum protection in protected environments, essential for the treatment of toxic and/or sterile substances.
3. In the ATMP and Regenerative Medicine sector, Comecer supplies equipment for the handling and treatment of Advanced Therapy Medicinal Products.
4. Finally, in the Services sector, Comecer offers a range of solutions that include assistance and maintenance, IoT production management software, and medical devices.
Joining the ATS Group has allowed the company to benefit from an international network and resources that enable it to access markets with a high degree of technology and expand into new sectors (such as high-speed packaging).
In any case, in whatever field it operates, Comecer not only brings design and technology but also a new way of working through its "Safety First" ethical code. This cornerstone principle permeates every aspect of corporate life and goes beyond current regulations, placing respect for the environment and the safety of people (a concept that also includes their well-being and training) at the center of every decision and corporate action.
Research and Development: The Frontier of Innovation

The ability to generate innovation and progress leads Comecer to constantly invest in research and development. In this area, the company collaborates with world-renowned universities and research centers and stands out for its commitment to creating systems for the treatment and prevention of non-communicable chronic diseases.
The Comecer Research & Development group is divided into various areas, each focused on a specific aspect: from mechanics to automation, from materials to chemistry. This multidisciplinary approach allows exploring new technological horizons and effectively responding to emerging challenges even in new fields.
An emblematic example of Comecer's ability to operate at unprecedented levels of precision and innovation is its participation in the ExoMars mission (a joint project of the European Space Agency and the Russian Space Agency Roscosmos), through the creation of an ultra-clean isolator developed in extremely clean conditions to avoid contamination.
Manufacturing footprint: quality, precision, and an integrated business cycle

And it is precisely in the ability to propose customized solutions that Comecer's value proposition lies. Combining craftsmanship and advanced technology, always in close connection with research and development centers, this approach is evident in the entire production process, from design to installation, ensuring maximum flexibility and constant control over quality.
The Comecer manufacturing process covers every phase of the business cycle, from initial design to production, from validation to installation, and to end-user training. This integrated cycle ensures that each product meets customer needs and the strict quality and safety standards required by the market, placing Comecer at the forefront of its sector.
Comecer products, a global success

For these reasons, Comecer's products are known worldwide and used in hospitals, universities, research centers, pharmaceutical companies, and large industrial groups. The flexibility in production and continuous investments in research and development allows Comecer to meet the specific needs of various sectors.
Among the standout products are:
- CLIO: A compact automatic dispenser for fractioning drugs into vials and syringes, suitable for hot cells.
- THEODORICO: A robotic system for the preparation of radiopharmaceutical doses in syringes and/or vials, reducing exposure to radiopharmaceuticals.
- ALCEO: An automated system for the synthesis and dispensing of radioactive tracers.
- IRIS: A system that automates dispensing and injection into vials or syringes.
- HELIOS: A fully automated dispensing system.
- FLEXYCULT: A modular incubator for cell therapy, equipped with a shared docking station, ideal for research.
- IBC: Workflow management software for nuclear medicine, based on client/server technology.
- MSTI: An isolator for sterility testing in aseptic environments.
- BABY PHILL: A filling line for vials, designed for clinical and development batches, suitable for sterile and cytotoxic drugs.
Every stage of the production process is subject to rigorous quality control. Products and systems are subjected to tests to ensure compliance with required specifications and current regulations. The tests include:
- Pre-FAT: Feasibility studies and preliminary integration tests.
- FAT: Practical demonstration of the system's operation in the presence of the customer.
- SAT: Tests conducted at the customer's facility to ensure system integrity.
- Integrated Quality Management System
Comecer also has an Integrated Management System for Quality, Environment, Safety, and Energy, compliant with all international standards.
Comecer grows in employment and communication
Despite the pandemic, Comecer has grown in recent years. In the three-year 2020-2022 (last available fiscal data), the company recorded a steady positive trend, with an increase in turnover in the radiopharmaceutical sector (+28.4%) and a strong recovery in the pharmaceutical sector (+7.0% compared to 2019/20). The Services sector also trended upward, with growth of 13.6%. These positive results are reflected in Comecer's planned activities (backlog), which saw an increase of 12.9% compared to pre-pandemic levels.
In a field like the mechanical sector, traditionally inclined to a higher male presence, Comecer stands out for its commitment to female inclusion and the development of new talents. 2023 has seen a significant hiring of female staff, which constitutes almost 16% of new entries (out of a total of 479 hires), signaling a paradigm shift in workforce composition.
The turnover rate (about 6%) indicates dynamic and proactive human resource management, confirming the company as a stimulating and continuously evolving environment.
This evolutionary process is also evident in the way the company is committed to external communication. The Marketing & Communication department of Comecer plays a crucial role in making the company's products and services known both through trade show communication (each year the company participates in about 40 international fairs) and digital communication (in 2023, the www.comecer.com website had an average of 200,000 visits per year). Social media channels LinkedIn, Facebook, Twitter, Instagram, and YouTube complete the communication offer.
Over the years, Comecer has demonstrated how to combine development in technologically advanced sectors, such as pharmaceutical and radiopharmaceutical, and the search for solutions that place patients and operators at the center of its interests. Its commitment to female inclusion and innovation in human resource management underscores its vocation for progress and diversity.
Comecer is an example of business success that combines innovation, social responsibility, and effective communication.
Fill out the form to download the PDF document
See all news »